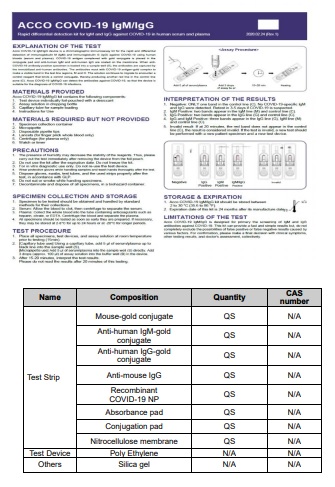
GDIH First Response in association with its group company AccoBiotechSdnBhd (Malaysia) has developed Rapid Diagnostic test kit for novel coronavirus COVID-19 infection. This test detects qualitatively both early marker and late marker, IgM/IgG antibodies of the novel coronavirus in human serum, plasma or whole blood in vitro.
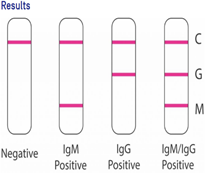
GDIH First Response-Acco COVID-19 IgM/IgG device is a chromatographic immunoassay kit for the rapid and differential detection of immunoglobulin M (lgM) and immunoglobulin G (IgG) against COVID-19 using serum, plasma and whole blood.

- Accurate Test Results: Within 15 minutes
- Sensitivity: 95% or more
- Specificity: 99%
- Rapid screening of carriers of virus that are symptomatic or asymptomatic
- Easy & Efficient: Intuitive visual interpretation, No special equipment needed
- Test Purpose: Clinical Diagnostic use, ideally suited for hospitals, clinics, laboratories
- GDIH First Response: R & D Facility
- First & Only Full Fledge RDT Manufacturing In Malaysia
- GMP Status, ISO certified, CE US Patent (No; 9,354,234)
- Recognize as BIOTECH company by BIOTECHCORP & MIDA
- Ministry of International Trade and Industry: Awarded Manufacturing License for “Diagnostic Test Kits”
- Diagnostic Kits:In vitro Diagnosis Test (IVD), Rapid Test Kit (RTK), Point of Care Test (POCT)
- Manufacturing Technology:Nano Technology
- Production Capacity:minimum of 30,000 kits per day
- Technology Innovations:Q-RTK & Microarray Chip etc
As discussed and understood with all stakeholders
- Competitors are offering spike protein based test kits. This is the same said rapid test kit which was used by wondfo etc cos and was supplied by ICMR to various states and then when kits had failed in giving results and all kits had been returned by various states to ICMR. Old story known to all of us.
- Our rapid test kit is antibody based kit which is nucleo based test kit which can better and accurate results. This is the reason we have qualified for USFDA and CE Europe.
- Important is also to mention that our R&D team is trying to work out membrane protein based rapid test kits which is still advanced and should be up in the markets shortly.
- We all know that COVID19 virus has a bizzare pattern of mutation (at least 8 such variants are known till date and more under path of discovery). Spike protein is a very normal chemical and can detect very very early stages of this virus so the results are inaccurate. However in our case nucleo protein base helps us detect advance mutations or RNAs of this virus. Accobiotech has been specialising in kits business for last several years and we stand committed to quality and assure our best products.
- Other competitors are mostly issuing and supplying kits basis certificate of conformity which is issued without a simple test and is just like applied for (A/F) license which has no valid credentials.
- We have been rigorously tested by the veteran institutions and hence we are time tested and stand committed to quality.
- All said and done accobiotech is working on real thin margins knowing a fact that the chemical used for the test kits are really expensive and yet our antibody rapid test kits have been priced sub-optimally just to serve the larger cause.
- The price which is being offered by competition has to have some rationality. If authorities wish to compare prices – they will have to do an Apple to Apple comparison – factored by few things like
- nucleo protein based test
- CE certificate (Europe) – very difficult to have.
- USFDA certificate (toughest certification) – very difficult to have.
- accuracy and specifity- basis results of the sample taken prior to issuing the certificates. In our case both have been 99.5% and 100%, respectively.
We would again like to submit that the price quoted by us are sub-optimal and rationally suited to fit to the times and larger cause.
We are there to assure quality of results. Rather focusing on price war we will have to again wait for the failure as happened in the past and then maybe supply but I am sure that it would be quite late by that time.
Enclosure –
Document on Rationale for certificate issuance.